America's FDA approves over-the-counter hearing aids
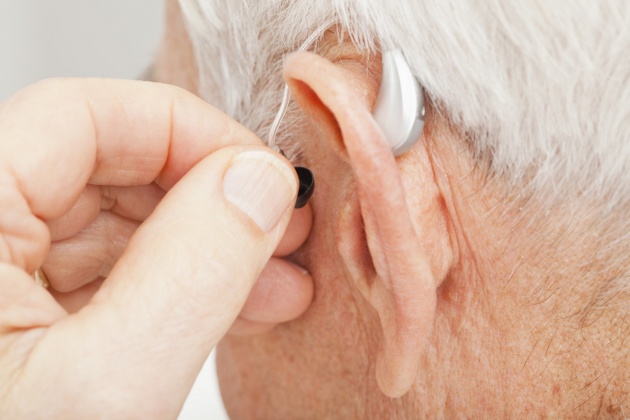
The FDA has cleared a direct-to-consumer hearing aid, developed by speaker manufacturer Bose, potentially opening the door for other consumer-focused electronics companies. The de novo clearance makes it the first hearing aid authorized by the agency that allows users to fit, program and control the device on their own, without help from a healthcare provider. The Bose Hearing Aid is a wireless amplifier placed in the ear canal that can be adjusted by the user through a smartphone app. In its review, the FDA evaluated clinical study data from 125 patients that showed outcomes with self-fitting of the Bose Hearing Aid were mostly comparable to professional fittings of the same device, between the amount of amplification selected, speech-in-noise testing and overall benefits. In addition, when participants self-fit the hearing aid, they reported generally preferring their settings over professionally selected settings. The agency describes hearing loss as a significant public health issue in an aging population and estimates that about 37.5 million adults have at least some trouble hearing. Though users may fit the hearing aid on their own, the Framingham, Massachusetts-based Bose must comply with applicable federal and state laws regarding the sale of hearing aids, including state laws that might require hearing aids to be purchased from or dispensed by a licensed hearing aid dispenser, the FDA said.